<Title:> The Cornea: An Ideal Tissue for Regenerative Medicine
<Author(s):> Shigeto Shimmura, Emi Inagaki, Masatoshi Hirayama, Shin Hatou
<Corresponding author E-Mill:> shige.shimmura(at)keio.jp
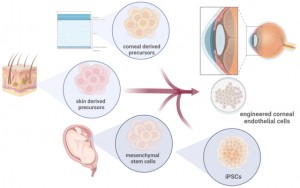
<Abstract:> Regenerative medicine is a highly anticipated field with hopes to provide cures for previously uncurable diseases such as spinal cord injuries and retinal blindness. Most regenerative medical products use either autologous or allogeneic stem cells, which may or may not be genetically modified. The introduction of induced-pluripotent stem cells (iPSCs) has fueled research in the field, and several iPSC-derived cells/tissues are currently undergoing clinical trials. The cornea is one of the pioneering areas of regenerative medicine, and already four cell therapy products are approved for clinical use in Japan. There is one other government-approved cell therapy product approved in Europe, but none are approved in the USA at present. The cornea is transparent and avascular, making it unique as a target for stem cell therapy. This review discusses the unique properties of the cornea and ongoing research in the field.
<Keywords:> cornea, stem cells, induced pluripotent stem cells, lacrimal gland, mesenchymal stem cells
<URL:> https://www.jstage.jst.go.jp/article/kjm/73/1/73_2023-0001-IR/_html

![Prevalence of Metabolic Syndrome and Lifestyle Characteristics by Business Type among Japanese Workers in Small- and Medium-sized Enterprises [Published online Keio J Med, 68, 54-67, by J-STAGE]](http://kjm.pupu.jp/blog/wp-content/uploads/2019/09/2018-0007-OA-100x100.jpg)
![Roles of Hypoxia Response in Retinal Development and Pathophysiology [Published online Keio J Med, 67, 1-9, by J-STAGE]](http://kjm.pupu.jp/blog/wp-content/uploads/2018/03/2017-0002-IR-100x100.jpg)
![Leadership Training of Surgery in US [Published online Keio J Med, 66, 54-54, by J-STAGE]](http://kjm.pupu.jp/blog/wp-content/uploads/2017/09/66-004-ABST-100x100.jpg)
![Stroke and the Cell Therapy Saga: Towards a Safe, Swift and Efficient Utilization of cells [Published online Keio J Med, 66, 55-55, by J-STAGE]](http://kjm.pupu.jp/blog/wp-content/uploads/2017/09/66-005-ABST-100x100.jpg)
![The History and Future of Unlinked Total Elbow Arthroplasty [Published online in advanced , by J-STAGE]](http://kjm.pupu.jp/blog/wp-content/uploads/2017/08/2017-0007-IR-100x100.jpg)
![Thrombosis in Lymphoma Patients and in Myeloma Patients [Published online Keio J Med, 64, 37-43, by J-STAGE]](http://kjm.pupu.jp/blog/wp-content/uploads/2015/10/kjm_2014-0017-RE1-100x100.jpg)
![Percutaneous Tendon Needling without Ultrasonography for Lateral Epicondylitis [Published online Keio J Med, 69, 37-42, by J-STAGE]](http://kjm.pupu.jp/blog/wp-content/uploads/2020/06/2019-0004-OA-100x100.jpg)
![Precision Cancer Medicine and Super-computing System [Published online Keio J Med, 66, 54-54, by J-STAGE]](http://kjm.pupu.jp/blog/wp-content/uploads/2017/09/66-003-ABST-100x100.jpg)
![Hospital Preparedness for COVID-19: The Known and The Known Unknown [Published online in advanced , by J-STAGE]](http://kjm.pupu.jp/blog/wp-content/uploads/2020/08/2020-0011-OA-100x100.jpg)
![A Theory of Diagnostic Testing to Stop the Virus Spreading: Evidence-based Reasoning to Resolve the COVID-19 Crisis by Testing [Published online in advanced , by J-STAGE]](http://kjm.pupu.jp/blog/wp-content/uploads/2022/02/2021-0009-IR-100x100.jpg)